Source: SOGC (click for the PDF)
BP Measurement
Recommendations
1. Blood pressure should be measured with the woman in the sitting position with the arm at the level of the heart. (II-2A)
2. An appropriately sized cuff (i.e., length 1.5 times the circumference of the arm) should be used. (II-2A)
3. Korotkoff phase V should be used to designate diastolic blood pressure. (I-A)
4. If blood pressure is consistently higher in one arm, the arm with the higher values should be used for all blood pressure measurements. (III-B)
5. Blood pressure can be measured using a mercury sphygmomanometer, a calibrated aneroid device, or an automated blood pressure machine that has been validated for use in preeclampsia. (II-2A)
6. Automated blood pressure machines that have not been validated for use in women with preeclampsia may underestimate or overestimate blood pressure in those women; a comparison of readings using mercury sphygmomanometry or a calibrated aneroid device is recommended. (II-2A)
7. In the office setting, when blood pressure elevation is non-severe and preeclampsia is not suspected, ambulatory blood pressure monitoring or home blood pressure monitoring is useful to confirm persistently elevated blood pressure. (II-2C)
8. When home blood pressure monitoring is used, maternity care providers should ensure that patients have adequate training in measuring their blood pressure and interpreting the readings. (III-C)
9. The accuracy of all blood pressure measurement devices used in hospitals or offices should be checked regularly against a calibrated device. (II-3C)
10. The accuracy of all automated devices used for home blood pressure monitoring should be checked regularly against a calibrated device. (III-C)
Diagnosis of Hypertension
Recommendations
11. The diagnosis of hypertension should be based on office or in-hospital blood pressure measurements. (II-B)
12. Hypertension in pregnancy should be defined as an office (or in-hospital) systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, based on the average of at least 2 measurements, taken at least 15 minutes apart, using the same arm. (II-2B)
13. Resistant hypertension should be defined as the need for 3 antihypertensive medications for blood pressure control at ≥ 20 weeks’ gestation. (III-C)
14. A transient hypertensive effect should be defined as an office systolic blood pressure ≥ 140 mmHg or a diastolic blood pressure ≥ 90 mmHg that is not confirmed after rest, on repeat measurement, on the same or on subsequent visits. (II-2B)
15. A white-coat hypertensive effect refers to blood pressure that is elevated in the office (i.e., systolic ≥ 140 mmHg or diastolic ≥ 90 mmHg), but < 135 mmHg (systolic) and < 85 mmHg (diastolic) on ambulatory or home blood pressure monitoring. (II-2B)
16. A masked hypertensive effect refers to blood pressure that is normal in the office (i.e., systolic < 140 mmHg and diastolic < 90 mmHg) but elevated on ambulatory or home blood pressure monitoring (i.e., systolic ≥ 135 mmHg or diastolic ≥ 85 mmHg). (II-2B)
17. Severe hypertension should be defined, in any setting, as a systolic blood pressure of ≥ 160 mmHg or a diastolic blood pressure of ≥ 110 mmHg based on the average of at least 2 measurements, taken at least 15 minutes apart, using the same arm. (II-2B)
18. All pregnant women should be assessed for proteinuria. (II-2B)
19. Urinary dipstick testing (by visual or automated testing) may be used for screening for proteinuria when the suspicion of preeclampsia is low. (II-2B)
20. Significant proteinuria should be defined as ≥ 0.3 g/d in a complete 24-hour urine collection or ≥ 30 mg/mmol urinary creatinine in a spot (random) urine sample. (II-2B)
21. Significant proteinuria should be suspected when urinary dipstick proteinuria is ≥ 1+. (II-2A)
22. More definitive testing for proteinuria (by urinary protein:creatinine ratio or 24-hour urine collection) is encouraged when there is a suspicion of preeclampsia, including: ≥ 1+ dipstick proteinuria in women with hypertension and risingblood pressure and in women withnormal blood pressure, but symptoms or signs suggestive of preeclampsia. (II-2A)
23. Proteinuria testing does not need to be repeated once significant proteinuria of preeclampsia has been confirmed. (II-2A)
24. There is insufficient information to make a recommendation about the accuracy of the urinary albumin:creatinine ratio. (II-2L)
Classification of HDPs
Recommendations
25. Hypertensive disorders of pregnancy should be classified as pre-existing hypertension, gestational hypertension, preeclampsia, or “other hypertensive effects” on the basis of different diagnostic and therapeutic considerations. (II-2B) (Table 2)
26. The presence or absence of preeclampsia must be ascertained, given its clear association with more adverse maternal and perinatal outcomes. (II-2B)
27. In women with pre-existing hypertension, preeclampsia should be defined as resistant hypertension, new or worsening proteinuria, one or more adverse conditions, or one or more severe complications. (II-2B)
28. In women with gestational hypertension, preeclampsia should be defined as new-onset proteinuria, one or more adverse conditions, or one or more severe complications. (II-2B)
29. Severe preeclampsia should be defined as preeclampsia complicated by one or more severe complications. (II-2B)
30. Severe preeclampsia, as defined in this guideline, warrants delivery. (II-2B)
31. The term PIH (pregnancy-induced hypertension) should be abandoned, as its meaning in clinical practice is unclear. (III-D)
Investigations to Classify HDPs
Recommendations
32. For women with pre-existing hypertension, the following should be performed in early pregnancy (if not previously documented): serum creatinine, fasting blood glucose, serum potassium, and urinalysis (III-D), and EKG. (II-2C)
33. Among women with pre-existing hypertension or those with a strong clinical risk marker for preeclampsia, additional baseline laboratory testing may be based on other considerations deemed important by health care providers. (III-C)
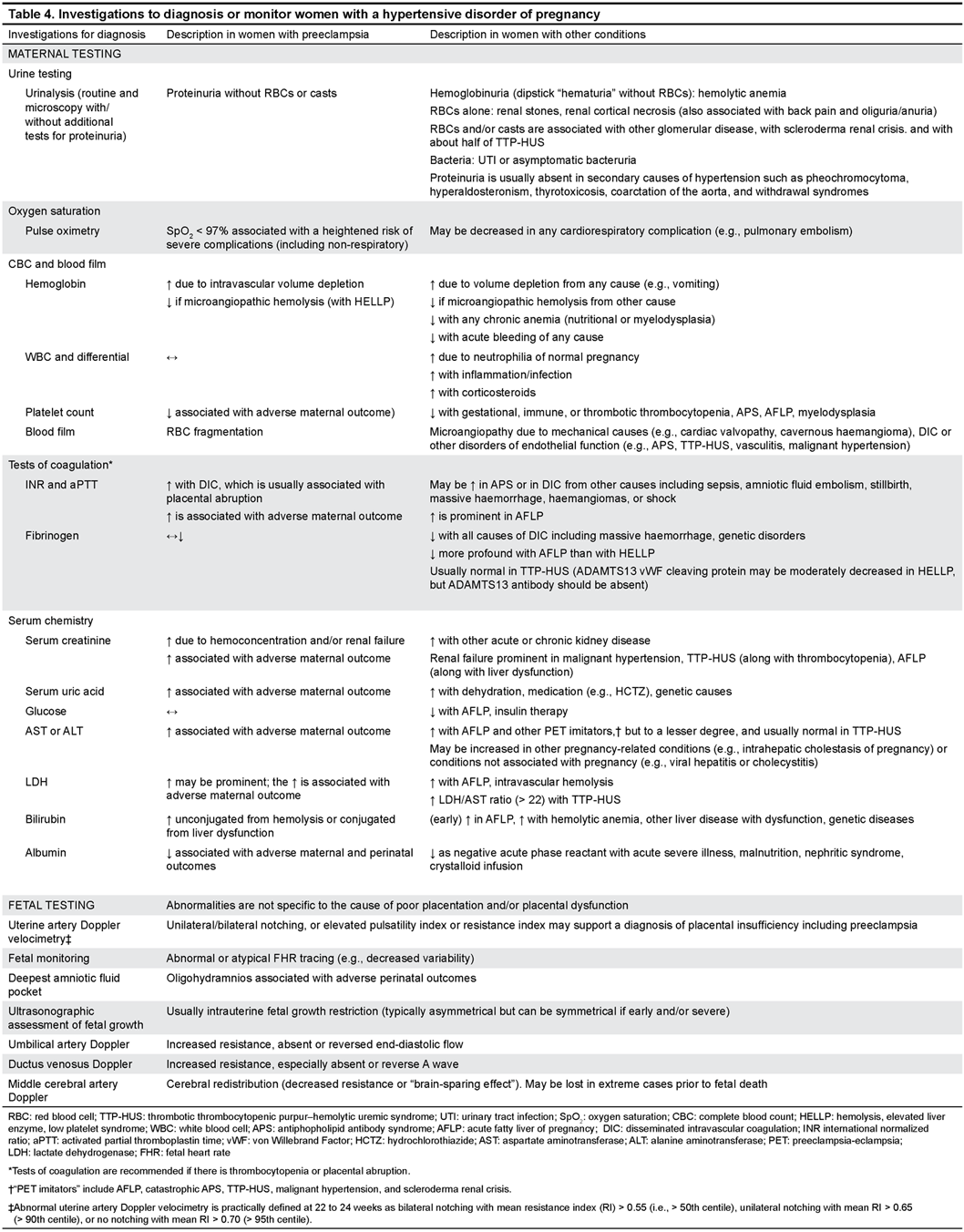
34. Women with suspected preeclampsia should undergo the maternal laboratory (II-2B) and pertinent fetal (II-1B) testing. (Table 4)
35. Doppler velocimetry-based assessment of the fetal circulation may be useful to support a placental origin for hypertension, proteinuria, and/or adverse conditions including intrauterine growth restriction, (II-2B) and for the timing of delivery. (I-A)
36. There is insufficient evidence to recommend use of the biophysical profile as part of a schedule of fetal testing in women with a hypertensive disorder of pregnancy. (II-2L)
37. If initial testing is reassuring, but there is ongoing concern about preeclampsia (e.g., change in maternal and/or fetal condition), maternal and fetal testing should be repeated. (III-C)
Investigations to Classify HDPs
Recommendations
32. For women with pre-existing hypertension, the following should be performed in early pregnancy (if not previously documented): serum creatinine, fasting blood glucose, serum potassium, and urinalysis (III-D), and EKG. (II-2C)
33. Among women with pre-existing hypertension or those with a strong clinical risk marker for preeclampsia, additional baseline laboratory testing may be based on other considerations deemed important by health care providers. (III-C)
34. Women with suspected preeclampsia should undergo the maternal laboratory (II-2B) and pertinent fetal (II-1B) testing. (Table 4)
35. Doppler velocimetry-based assessment of the fetal circulation may be useful to support a placental origin for hypertension, proteinuria, and/or adverse conditions including intrauterine growth restriction, (II-2B) and for the timing of delivery. (I-A)
36. There is insufficient evidence to recommend use of the biophysical profile as part of a schedule of fetal testing in women with a hypertensive disorder of pregnancy. (II-2L)
37. If initial testing is reassuring, but there is ongoing concern about preeclampsia (e.g., change in maternal and/or fetal condition), maternal and fetal testing should be repeated. (III-C)
Predicting Preeclampsia
Recommendations
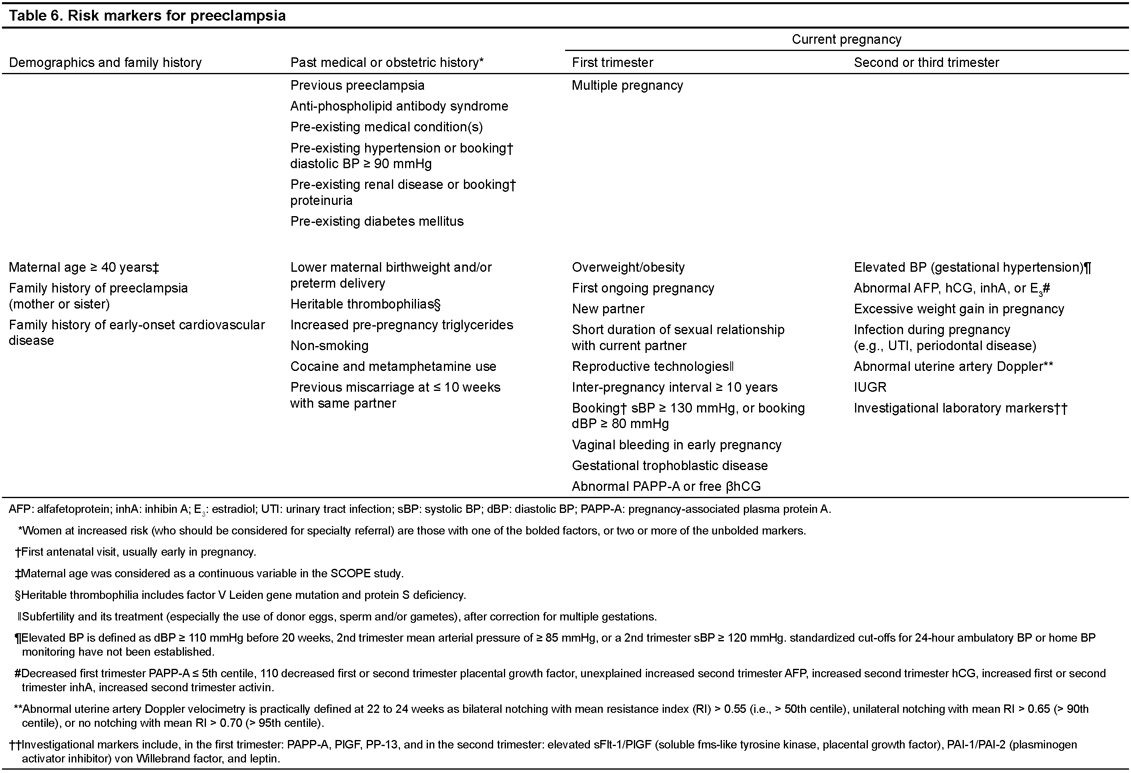
38. Women should be screened for clinical risk markers of preeclampsia from early pregnancy. (II-2C) (Table 6)
39. Consultation with an obstetrician or an obstetric internist, by telephone if necessary, should be considered for women with a history of previous preeclampsia or another strong clinical marker of increased preeclampsia risk, particularly multiple pregnancy, antiphospholipid antibody syndrome, significant proteinuria at first antenatal visit (usually early in pregnancy), or a pre-existing condition of hypertension, diabetes mellitus, or renal disease. (II-2B)
40. Screening using biomarkers or Doppler ultrasound velocimetry of the uteroplacental circulation cannot be recommended routinely at present for women at low or increased risk of preeclampsia until such screening has been shown to improve pregnancy outcome. (II-2C)
Preventing Preeclampsia and its Complications in Women at Low Risk
Recommendations
41. Calcium supplementation of at least 1 g/d, orally, is recommended for women with low dietary intake of calcium (< 600 mg/d). (I-A)
42. The following are recommended for other established beneficial effects in pregnancy: abstention from alcohol for prevention of fetal alcohol effects (II-2E), exercise for maintenance of fitness (I-A), periconceptual use of a folate-containing multivitamin for prevention of neural tube defects (I-A), and smoking cessation for prevention of low birthweight and preterm birth. (I-E)
43. Periconceptual and ongoing use of a folatecontaining multivitamin (I-B) or exercise (II-2B) may be useful in preventing preeclampsia.
44. Prostaglandin precursors and supplementation with magnesium or zinc are not recommended for preeclampsia prevention, but may be useful for prevention of other pregnancy complications. (I-C)
45. Dietary salt restriction during pregnancy (I-D), calorie restriction during pregnancy for overweight women (I-D), low-dose acetylsalicylic acid (I-E), vitamins C and E (based on current evidence) (I-E), and thiazide diuretics (I-E) are not recommended.
46. There is insufficient evidence to make a recommendation about a heart-healthy diet (II-2L); workload or stress reduction (including bedrest) (II-2L); supplementation with iron with or without folate (I-L); vitamin D (I-L); pyridoxine (I-L); or food rich in flavonoids. (I-L)
Preventing Preeclampsia and itsComplications in Women at Increased Risk
Recommendations
47. Low-dose acetylsalicylic acid and calcium supplementation (of at least 1 g/d) for women with low calcium intake are recommended for preventions of preeclampsia in women at high risk. (I-A)
48. Acetylsalicylic acid should be: taken in a low dose (75–162 mg/d), (III-B) administered at bedtime, (I-B) initiated after diagnosis of pregnancy but before 16 weeks’ gestation, (I-B) and considered for continuation until delivery. (I-C)
49. Prophylactic doses of low-molecular-weight heparin may be discussed in women with previous placental complications (including preeclampsia) to prevent the recurrence of severe or early-onset preeclampsia, preterm delivery, and/or infants that are small for gestational age. (I-B)
50. The following may be useful: L-arginine (I-B), increased rest at home in the third trimester (I-C), and reduction of workload or stress. (III-C)
51. The following may be useful for prevention of other pregnancy complications: prostaglandin precursors (I-B), magnesium supplementation (I-C), and heparin to prevent venous thromboembolic disease. (I-B)
52. The following are recommended for other established beneficial effects in pregnancy (as discussed for women at low risk of preeclampsia): abstention from alcohol (II-2E), periconceptual use of a folate-containing multivitamin (I-A), and smoking cessation. (I-E)
53. The following are not recommended: calorie restriction in overweight women during pregnancy (I-D), weight maintenance in obese women during pregnancy (III-D), antihypertensive therapy specifically to prevent preeclampsia (I-D), and vitamins C and E. (I-E)
54. There is insufficient evidence to make a recommendation about the usefulness of the following: the heart-healthy diet (III-L); exercise (I-L); selenium (I-L); garlic (I-L); zinc, pyridoxine, iron (with or without folate), vitamin D, or multivitamins with/without micronutrients. (III-L)
Dietary and Lifestyle Changes
Recommendations
55. There is insufficient evidence to make a recommendation about the usefulness of the following: new severe dietary salt restriction forwomen with any HDP, ongoing salt restriction among women with pre-existing hypertension, heart-healthy diet, and calorie restriction for obese women. (III-L)
56. There is insufficient evidence to make a recommendation about the usefulness of exercise, workload reduction, or stress reduction. (III-L)
57. For women with gestational hypertension (without preeclampsia), some bed rest in hospital (vs. unrestricted activity at home) may be useful to decrease severe hypertension and preterm birth. (I-B)
58. For women with preeclampsia who are hospitalized, strict bed rest is not recommended. (I-D)
59. For all other women with an HDP, the evidence is insufficient to make a recommendation about the usefulness of some bed rest, which may nevertheless be advised based on practical considerations. (III-C)
Place of Care
Recommendations
60. In-patient care should be provided for women with severe hypertension or severe preeclampsia. (II-2B).
61. A component of care through hospital day units or home care can be considered for women with nonsevere preeclampsia or non-severe pre-existing or gestational hypertension. (I-B, II-2B)
Antihypertensive Therapy for Severe Hypertension
Recommendations
62. Blood pressure should be lowered to < 160 mmHg systolic and < 110 mmHg diastolic. (I-A)
63. Initial antihypertensive therapy in the hospital setting should be with nifedipine short-acting capsules, parenteral hydralazine, or parenteral labetalol. (I-A) (Table 7)
64. Alternative antihypertensive medications include a nitroglycerin infusion (I-B), oral methyldopa (I-B), oral labetalol (I-B), oral clonidine (III-B), or postpartum, oral captopril. (III-B)
65. Refractory hypertension may be treated with sodium nitroprusside. (III-B)
66. Nifedipine and magnesium sulphate can be used contemporaneously. (II-2B)
67. Magnesium sulphate is not recommended solely as an antihypertensive agent. (I-E)
68. Continuous fetal heart rate monitoring is advised until blood pressure is stable. (III-L)
Antihypertensive Therapy for Non-Severe Hypertension Without Comorbid Conditions
Recommendations
69. Antihypertensive drug therapy may be used to keep systolic blood pressure at 130 to 155 mmHg and diastolic blood pressure at 80–105 mmHg. (I-B)
70. The choice of antihypertensive agent for initialtreatment should be based on characteristics of thepatient, contraindications to a particular drug, and physician and patient preference. (III-C)
71. Initial therapy in pregnancy can be with one ofa variety of antihypertensive agents available inCanada: methyldopa (I-A), labetalol (I-A), othe rbeta-blockers (acebutolol, metoprolol, pindolol, andpropranolol), (I-B) and calcium channel blockers(nifedipine). (I-A) (Table 8)
72. Angiotensin-converting enzyme inhibitors andangiotensin receptor blockers should not be used during pregnancy. (II-2E)
73. Atenolol and prazosin are not recommended prior to delivery. (I-D)
For Non-Severe Hypertension (BP of 140–159/90–109 mmHg) With Comorbid Conditions
Recommendations
74. For women with comorbid conditions, antihypertensive drug therapy should be used to keep systolic blood pressure at < 140 mmHg and diastolic blood pressure at < 90 mmHg. (III-C)
75. Initial therapy in pregnancy can be with one of a variety of antihypertensive agents as listed for women without co-morbidities. (III-C)
76. Captopril, enalapril, or quinapril may be used postpartum, even during breastfeeding. (III-B)
Corticosteroids for Acceleration of Fetal Pulmonary Maturity
Recommendations
77. Antenatal corticosteroid therapy should be considered for all women who present with preeclampsia at ≤ 34+6 weeks’ gestation. (I-A)
78. Antenatal corticosteroid therapy should be considered for women who present at ≤ 34+6 weeks’ gestation with gestational hypertension (despite the absence of proteinuria or adverse conditions) only if delivery is contemplated within the next 7 days. (III-L)
79. A rescue dose of corticosteroids may be considered for women at ≤ 34+6 weeks’ gestation who remain at high risk of preterm delivery 7 days or more after an initial course of antenatal corticosteroids. (I-C)
80. Antenatal corticosteroids may be considered for women delivered by elective Caesarean delivery at ≤ 38+6 weeks’ gestation to reduce respiratory morbidity. (I-B)
Timing of Delivery for Women With Preeclampsia
Recommendations
81. Consultation with an obstetrician (by telephone if necessary) is mandatory in women with severe preeclampsia. (III-B)
82. All women with severe preeclampsia should be delivered immediately (either vaginally or by Caesarean), regardless of gestational age. (III-C)
83. For women with non-severe preeclampsia at < 24+0 weeks’ gestation, counselling should include, as an option, information about delivery within days. (II-2B)
84. For women with non-severe preeclampsia at 24+0 to 33+6 weeks’ gestation, expectant management should be considered, but only in perinatal centres capable of caring for very preterm infants. (I-B)
85. For women with non-severe preeclampsia at 34+0 to 36+6 weeks’ gestation, there is insufficient evidence to make a recommendation about the benefits or risks of expectant management. (III-L)
86. For women with preeclampsia at ≥ 37+0 weeks’ gestation, immediate delivery is recommended. (I-A)
87. For women with non-severe preeclampsia complicated by hemolysis, elevated liver enzymes, low platelets syndrome at 24+0 to 34+6 weeks’ gestation, consider delaying delivery long enough to administer antenatal corticosteroids for acceleration of fetal pulmonary maturity if there is temporary improvement in maternal laboratory testing. (II-2B)
88. All women with hemolysis, elevated liver enzymes, low platelets syndrome at ≥ 35+0 weeks’ gestation should be considered for immediate delivery. (II-2B)
Timing of Delivery for Women With Gestational Hypertension
Recommendations
89. For women with gestational hypertension (without preeclampsia) at ≥ 37+0 weeks’ gestation, delivery within days should be discussed. (I-B)
90. For women with gestational hypertension (without preeclampsia) at < 37+0 weeks’ gestation, there is insufficient evidence to make a recommendation about the benefits or risks of expectant management. (III-L)
Timing of Delivery for Women With Pre-Existing Hypertension
Recommendation
91. For women with uncomplicated pre-existing hypertension who are otherwise well at ≥ 37+0 weeks’ gestation, delivery should be considered at 38+0 to 39+6 weeks’ gestation. (II-1B)
Mode of Delivery
Recommendations
92. For women with any hypertensive disorder of pregnancy, vaginal delivery should be considered unless a Caesarean delivery is required for the usual obstetric indications. (II-2B)
93. If vaginal delivery is planned and the cervix is unfavourable, then cervical ripening should be used to increase the chance of a successful vaginal delivery. (1-A)
94. At a gestational age remote from term, women with a hypertensive disorder of pregnancy with evidence of fetal compromise may benefit from delivery by emergency Caesarean section. (II-2B)
95. Antihypertensive treatment should be continued throughout labour and delivery to maintain systolic blood pressure at < 160 mmHg and diastolic blood pressure at < 110 mmHg. (II-2B)
96. The third stage of labour should be actively managed with oxytocin, 5 units intravenous or 10 units intramuscular, particularly in the presence of thrombocytopenia or coagulopathy. (I-A)
97. Ergometrine maleate should not be administered to women with any hypertensive disorder of pregnancy, particularly preeclampsia or gestational hypertension; alternative oxytocics should be considered. (II-3D)
Anaesthesia: General Principles
Recommendations
98. The anaesthesiologist should be informed when a woman with preeclampsia is admitted to the delivery suite. (II-3B)
99. Early insertion of an epidural catheter (in the absence of contraindications) is recommended for control of labour pain. (I-A)
100. In the absence of contraindications, all of the following are acceptable methods of anaesthesia for women undergoing Caesarean delivery: epidural, spinal, combined spinal-epidural, and general anaesthesia. (I-A)
101. A routine fixed intravenous fluid bolus should not be administered prior to neuraxialanaesthesia. (I-E)
Anaesthesia: Fluid Administration
Recommendations
102. Intravenous and oral fluid intake should be minimized in women with preeclampsia, to avoid pulmonary edema. (II-2B)
103. Fluid should not be routinely administered to treat oliguria (< 15 mL/hr for 6 consecutive hours). (III-D)
104. For treatment of persistent oliguria, neither dopamine nor furosemide is recommended. (I-E)
105. Phenylephrine or ephedrine may be used to prevent or treat hypotension during neuraxial anaesthesia. (I-A)
Monitoring
Recommendations
106. Arterial line insertion may be used for continuous arterial blood pressure monitoring when blood pressure control is difficult or there is severe bleeding. (II-3B)
107. Central venous pressure monitoring is not routinely recommended, and if a central venous catheter is inserted, it should be used to monitor trends and not absolute values. (II-2D)
108. Pulmonary artery catheterization is not recommended unless there is a specific associated indication (III-D), and then only in an intensive care unit setting. (III-B)
Coagulation
Recommendations
109. Upon admission to delivery suite, women with preeclampsia should have a platelet count done. (II-1A)
110. Neuraxial analgesia and/or anaesthesia are appropriate in women:
a. with preeclampsia, provided there are no associated coagulation concerns (II-2E) (Table 9);
b. with a platelet count ≥ 75 × 109/L, (II-2B);
c. taking low-dose acetylsaliclylic acid in the presence of an adequate platelet count (I-A);
d. receiving unfractionated heparin in a dose of ≤ 10 000 IU/d subcutaneously, 4 hours after the last dose and possibly immediately after the last dose without any delay (III-B);
e. receiving unfractionated heparin in a dose > 10 000 IU/d subcutaneously if they have a normal activated partial thromboplastin time 4 hours after the last dose (III-B);
f. receiving intravenous heparin in a therapeutic dose if they have a normal activated partial thromboplastin time 4 hours after the last dose (III-B); or
g. receiving low-molecular-weight heparin 10 to 12 hours after a prophylactic dose, or 24 hours after a therapeutic dose. (III-B)
Aspects of Care Specific to Women With Pre-Existing Hypertension
Recommendations
111. Pre-conceptual counselling for women with pre-existing hypertension is recommended. (III-C)
112. The following antihypertensive drugs are all acceptable for use in the first trimester of pregnancy: methyldopa, labetalol, and nifedipine. (II-2B)
113. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers should be discontinued when planning pregnancy or as soon as pregnancy is diagnosed. (II-2D)
114. Atenolol should be discontinued when pregnancy is diagnosed. (I-D)
115. Planned changes in antihypertensive agent(s) for care in pregnancy should be made while the woman is planning pregnancy if the woman has uncomplicated pre-existing hypertension, or, if in the presence of comorbid conditions, she is likely to conceive easily (within 12 months). (III-L)
Aspects of Care for Women With Preeclampsia: Magnesium Sulphate for Preventing or Treating Eclampsia
Recommendations
116. Magnesium sulphate is recommended for first-line treatment of eclampsia. (I-A)
117. Magnesium sulphate is recommended as prophylaxis against eclampsia in women with severe preeclampsia. (I-A)
118. Magnesium sulphate may be considered as prophylaxis against eclampsia in women with non-severe preeclampsia but with severe hypertension, headaches/visual symptoms, right upper quadrant/epigastric pain, platelet count < 100 000 × 109/L, progressive renal insufficiency, and/or elevated liver enzymes, based on cost considerations. (I-C)
119. Magnesium sulphate should be used in standard dosing, usually 4 g intravenous loading dose followed by 1 g/hour. (I-A)
120. Routine monitoring of serum magnesium levels is not recommended. (I-E)
121. Phenytoin and benzodiazepines should not be used for eclampsia prophylaxis or treatment, unless there is a contraindication to magnesium sulphate or it is ineffective. (I-E)
122. In women with pre-existing or gestational hypertension, magnesium sulphate should be considered for fetal neuroprotection in the setting of imminent preterm birth (within the next 24 hours) at ≤ 31+6 weeks. (1-A)
123. Delivery should not be delayed in order to administer antenatal magnesium sulphate for fetal neuroprotection if there are maternal and/or fetal indications of emergency delivery. (III-E)
Aspects of Care for Women With Preeclampsia: Plasma Volume Expansion
Recommendation
124. Plasma volume expansion is not recommended for women with preeclampsia. (I-E)
Therapies for HELLP Syndrome
Recommendations
125. Every obstetrical centre should be aware of the local delay between ordering and receiving platelets units. (III-B)
126. For a platelet count of < 20 × 109/L with hemolysis, elevated liver enzymes, low platelets syndrome, platelet transfusion is recommended regardless of mode of delivery. (III-B) (Table 9)
127. For a platelet count of 20 × 109 to 49 × 109/L with hemolysis, elevated liver enzymes, low platelets syndrome, platelet transfusion is recommended prior to Caesarean delivery. (III-B) (Table 9)
128. For a platelet count of 20 × 109 to 49 × 109/L with hemolysis, elevated liver enzymes, low platelets syndrome, platelet transfusion should be considered prior to vaginal delivery if there is excessive active bleeding, known platelet dysfunction, a rapidly falling platelet count, or coagulopathy. (II-2D) (Table 10)
129. For a platelet count of ≥ 50 × 109/L with hemolysis, elevated liver enzymes, low platelets syndrome, platelet transfusion and/or packed red blood cells should be considered prior to either Caesarean or vaginal delivery only if there is excessive active bleeding, known platelet dysfunction, a rapidly falling platelet count, or coagulopathy. (III-B)
130. We do not recommend corticosteroids for treatment of hemolysis, elevated liver enzymes, low platelets syndrome until they have been proven to decrease maternal morbidity. (II-3L)
131. We recommend against plasma exchange or plasmapheresis for hemolysis, elevated liver enzymes, low platelets syndrome, particularly within the first 4 days postpartum. (II-3E)
132. Blood pressure should be measured during the time of peak postpartum blood pressure, at days 3 to 6 after delivery. (III-B)
133. Women with postpartum hypertension should be evaluated for preeclampsia (either arising de novo or worsening from the antenatal period). (II-2B)
134. Consideration should be given to continuing antihypertensive therapy postpartum, particularly in women with antenatal preeclampsia and those who delivered preterm. (II-2L)
135. Severe postpartum hypertension must be treated with antihypertensive therapy to keep systolic blood pressure < 160 mmHg and diastolic blood pressure < 110 mmHg. (I-A)
136. In women without co-morbidities, antihypertensive therapy should be considered to treat non-severe postpartum hypertension to keep blood pressure < 140/90 mmHg. (III-L)
137. Women with co-morbidities other than pre-gestational diabetes mellitus should be treated to keep blood pressure < 140/90 mmHg (III-C)
138. Women with pre-gestational diabetes mellitus should be treated to keep blood pressure < 130/80 mmHg. (III-C)
139. Antihypertensive agents generally acceptable for use in breastfeeding include the following: nifedipine XL, labetalol, methyldopa, captopril, and enalapril. (III-B)
140. There should be confirmation that end-organ dysfunction of preeclampsia has resolved. (III-C)
141. Non-steroidal anti-inflammatory drugs should not be given postpartum if hypertension is difficult to control, there is evidence of kidney injury (oliguria and/or creatinine≥ 90 μM), or platelets are < 50 to 109/L. (III-C)
142. Postpartum thromboprophylaxis should be considered in women with preeclampsia, particularly in the presence of other risk factors. (II-2B)
Care Beyond 6 Weeks Postpartum
Recommendations
143. Women with a history of severe preeclampsia (particularly those who presented or delivered before 34 weeks’ gestation) should be screened for pre-existing hypertension and underlying renal disease. (II-2B)
144. Referral for internal medicine or nephrology consultation (by telephone if necessary) should be considered for women with:
(i) postpartum hypertension that is difficult to control, or
(ii) women who had preeclampsia and have at 3-6 months postpartum either ongoing proteinuria, decreased estimated glomerular filtration rate (eGFR) (< 60 mL/min), or another indication of renal disease, such as abnormal urinary sediment. (III-A)
145. Women who are overweight should be encouraged to attain a healthy body mass index to decrease risk in future pregnancy (II-2A) and for long-term health. (I-A)
146. Women with pre-existing hypertension or persistent postpartum hypertension should undergo the following investigations (if not done previously) at least six weeks postpartum: urinalysis; serum sodium, potassium and creatinine; fasting glucose; fasting lipid profile; and standard 12-lead electrocardiography. (III-L)
147. Women who are normotensive but who have had a hypertensive disorder of pregnancy, may benefit from assessment of traditional cardiovascular risk markers. (II-2B)
148. All women who have had a hypertensive disorder of pregnancy should pursue a healthy diet and lifestyle. (I-B)
Effects of Maternal Hypertension and Its Therapies on Child Neurobehavioural Development
Recommendations
149. Clinicians should be aware that gestational hypertension and preeclampsia may each be associated with an increase in adverse paediatric neurodevelopmental effects, such as inattention and externalizing behaviours (e.g., aggressiveness). (II-2B).
150. Clinicians should be reassured that there is no compelling evidence that antihypertensive medications (specifically labetalol, nifedipine, or methyldopa) are themselves associated with clear adverse neurodevelopmental effects. (I-B)
PATIENT PERSPECTIVE
Recommendations
151. Health care providers should be alert to symptoms of posttraumatic stress following a hypertensive disorder of pregnancy and refer women for appropriate evaluation and treatment. (II-2B)
152. Health care providers should inform their patients, antepartum and postpartum, about preeclampsia, its signs and symptoms, and the importance of timely reporting of symptoms to health care providers. (II-2B)
153. Information should be re-emphasized at subsequent visits. (III-C)